Polycythemia vera (PV) is the most common type of Philadelphia-negative myeloproliferative neoplasms (MPNs). PV is a long-term debilitating and life-threatening condition as it is associated with the risk of thrombosis, hemorrhage, and a long-term propensity to develop myelofibrosis and secondary acute myeloid leukemia. Early intervention with disease-modifying treatment that can eradicate the mutated clones is needed to prevent disease progression and transformation. To date, therapeutic options for PV are limited and no cure is available. Most low-risk patients with PV are treated with phlebotomy and low-dose aspirin to manage increased blood viscosity and reduce the risk of vascular events, whereas high-risk patients require cytoreductive treatment. Several cytoreductive agents are available and hydroxyurea (HU) is commonly used as a first-line therapy. Ruxolitinib, a JAK2 inhibitor, is approved as a second-line treatment of PV restricted to patients who are resistant to or intolerant of HU. While interferon alfa agents have reported efficacy in patients with PV, their use has been limited by tolerability concerns.
Ropeginterferon alfa-2b (P1101), a long-acting, mono-PEGylated proline interferon, was developed for the treatment of PV. The novel formulation of P1101 improves its pharmacokinetic properties allowing once every 2 weeks administration and offering improved tolerability and convenience. P1101 was evaluated for the treatment of PV in the phase 3 PROUD-PV trial and its extension study CONTINUATION-PV. In CONTINUATION-PV, a significantly higher proportion of patients treated with P1101 achieved complete hematologic response (CHR) with improved disease burden at 36 months compared with patients treated with HU. P1101 has recently been approved by the European Medicines Agency and the Taiwan Food and Drug Administration as monotherapy in adults for the treatment of PV without symptomatic splenomegaly. Here, we describe a phase 2 study to evaluate the safety and efficacy of P1101 in Japanese patients with PV for whom the current standard of treatment is difficult to apply.
This is an ongoing phase 2, open-label, multicenter, single-arm study. Eligible patients receive subcutaneous administration of P1101, starting at 100 μg with dose escalation by 50 μg every 2 weeks until stabilization of hematological parameters at the highest tolerated dose. The dose is maintained at the highest dose that can be tolerated and delivers the best disease response. The maximum recommended single dose is 500 μg every 2 weeks. Low-dose aspirin is administered during the 52 weeks of study treatment, unless contraindicated. At weeks 36 and 52, the primary study endpoint, phlebotomy-free CHR, is analyzed. Secondary endpoints include changes in hematocrit, white blood cell count, platelet count, and spleen size from baseline; time requiring no phlebotomy; time to first response; and duration of response maintenance. After completion of 52-weeks of treatment, collection of the long-term follow-up information (blood parameters, molecular and cytogenetic data, safety parameters, and optional bone marrow data) will be continued until the drug becomes commercially available for all patients.
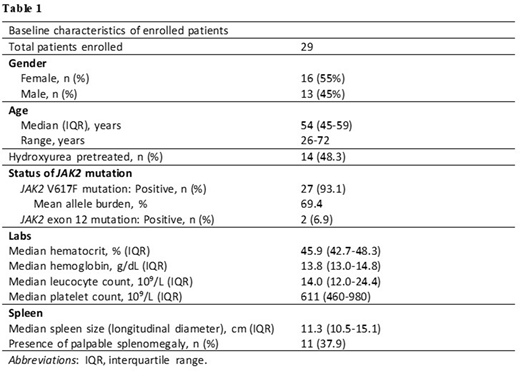
Key eligibility criteria include patients diagnosed with PV according to the WHO criteria, a total cumulative HU treatment duration < 3 years at screening, and patients where the current standard therapy is difficult to apply. The study population includes patients across a wide age range (26 to 72 years) with the median age of 54. Patients are 55% female, 48.3% received prior HU therapy, and 93.1% are JAK2 V617F mutation positive. Patients with a documented history of refractoriness to HU, previous use of interferon alfa, or symptomatic splenomegaly are not eligible. Additional baseline characteristics are shown in Table 1.
This study addresses an unmet need for patients where standard therapy cannot be applied. The study population includes younger patients who would require treatment, patients who are classified as low risk of thrombosis, but are considered to be candidates for cytoreductive therapy based on disease-related symptoms, and patients who are intolerant to HU. Data from these patients will be used to demonstrate the safety and efficacy of P1101 and help patients for whom the standard of care is difficult to apply. Trial results are anticipated for Q3 2021. NCT04182100.
Kirito:Novartis Pharma KK: Honoraria; Shire Japan: Honoraria; Pharmaessential Japan: Honoraria. Shimoda:Otsuka Pharmaceutical: Research Funding; Asahi Kasei Medical: Research Funding; Japanese Society of Hematology: Research Funding; The Shinnihon Foundation of Advanced Medical Treatment Research: Research Funding; Pfizer Inc.: Research Funding; Kyowa Hakko Kirin Co., Ltd.: Research Funding; CHUGAI PHARMACEUTICAL CO., LTD.: Research Funding; Merck & Co.: Research Funding; Astellas Pharma: Research Funding; AbbVie Inc.: Research Funding; PharmaEssentia Japan: Research Funding; Perseus Proteomics: Research Funding; Celgene: Honoraria; Shire plc: Honoraria; Bristol-Myers Squibb: Honoraria; Takeda Pharmaceutical Company: Honoraria; Novartis: Honoraria, Research Funding. Komatsu:Otsuka Pharmaceutical Co., Ltd., Shire Japan KK, Novartis Pharma KK, PharmaEssentia Japan KK, Fuso Pharmaceutical Industries, Ltd., Fujifilm Wako Pure Chemical Corporation, Chugai Pharmaceutical Co., Ltd., Kyowa Hakko Kirin Co., Ltd., Takeda Pharmaceutica: Research Funding; Meiji Seika Pharma Co., Ltd.: Patents & Royalties: PCT/JP2020/008434, Research Funding; Takeda Pharmaceutical Co., Ltd, Novartis Pharma KK, Shire Japan KK: Speakers Bureau; AbbVie: Other: member of safety assessment committee in M13-834 clinical trial.; PPMX: Consultancy, Research Funding; Otsuka Pharmaceutical Co., Ltd., PharmaEssentia Japan KK, AbbVie GK, Celgene KK, Novartis Pharma KK, Shire Japan KK, Japan Tobacco Inc: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.